The PiVOTal Difference
PiVOT starts with ‘pi’, a value as precise and limitless as their capability to help clients, enabling them to execute clinical research with validity, optimization, and transparency. Our Network Partner's expertise with site management organization and clinical trial support services constitutes the turning point that transforms your trials into triumphs.

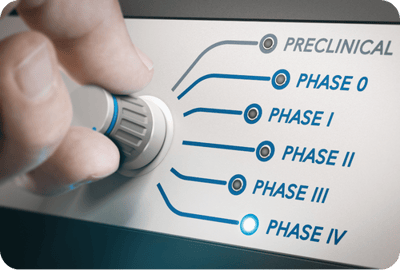
Drug and Device Trial Management
PiVOT manages Phase I to IV trials for drugs, biologics, and devices, focusing on proof of concept or small-scale studies. Services encompass protocol development, regulatory affairs, monitoring, data management and analysis, and report writing. Ensure compliance with Data Privacy Laws, Good Clinical Practice, and relevant regulatory guidelines. PiVOT strives to support publication in peer-reviewed journals or facilitate decisions on advancing to large-scale FDA registration trials.Post Marketing Surveillance (PMS) and Real World Evidence (RWE)
PiVOT provides comprehensive support for Post Marketing Surveillance (PMS) and Real World Evidence (RWE) studies, from pre-regulatory stages to study close-out and data analysis reporting. Services ensure compliance with Data Privacy Laws, Good Clinical Practice, and relevant regulatory guidelines. They cover protocol development, monitoring, data management and analysis, and report writing for seamless execution of studies.
PiVOT's Site Management Organization (SMO)
Their SMO platform provides contract research organizations (CROs), pharmaceutical and biotechnology companies, medical device companies, and especially clinical investigation sites – critical partners – with excellent clinical-trial-related services, including:
PiVOT By The Numbers
23
Sites
16
Studies
40,000+
Participants Recruited

Get your time back and let us find the solutions you need!
GLSA’s Global Network of solutions incorporates a variety of CROs and specialty vendors to support the specific needs of your clinical trial. Our network includes full-service CROs, biometrics, site selection, patient recruitment and retention, clinical supply chain management, and more. GLSA pre-qualifies all members of our network so you can be comfortable working with quality service providers.
We connect Sponsors with the CRO that has the right experience, culture, and capabilities to execute their research protocol. We leverage our extensive experience and harness our key relationships to work for CROs. We can fill any gaps you need to support your client’s trials. GLSA takes the guesswork out of vendor selection with our industry experts and experience.
What are your challenges? Contact GLSA to learn more about how we can help you accelerate your speed to market.